We’re happy to share the recording of the most recent session of our webinar series, Sharing Knowledge, Solving Problems. During this interactive Q&A session, Dr. Naveen Agarwal fielded questions surrounding ISO 14971 with topics including risk analysis, ICH Q9, FMEA, PHA, and more. We’re thrilled to see the community coming together to discuss questions and obstacles in the risk management industry. This dialogue is key to developing understanding and finding solutions to future challenges.
Read MoreA double-blinded, placebo-controlled, randomized clinical trial provides support for FDA approval of Remdesivir for treating COVID-19. The FDA has just approved a new antiviral drug, Remdesivir from Gilead Sciences, for treating COVID-19 patients needing acute care in a hospital. This treatment was available until now under an Emergency Use Authorization (EUA), but a full FDA approval will facilitate broader use.
Read MoreRecently, several treatment options have become available to treat the most seriously ill COVID-19 patients. Antivirals such as Remdesivir and corticosteroids such as Dexamethasone are now being used to improve the recovery time and mortality rate among hospitalized patients. However, their curative effect against COVID-19 has not yet been established and these treatments are not widely available to the public.
Read MoreOne of the challenges in risk analysis of medical devices is the diversity of use scenarios based on the target medical purpose and use environments. That is why ISO 14971:2019, the recently revised International Standard for Risk Management of Medical Devices, requires that risk analysis begin with a clear understanding of the intended use and reasonably foreseeable misuse.
Read MoreRisk analysis is a key requirement of ISO 14971:2019, the recently revised International Standard for Risk Management of Medical Devices. As outlined in Clause 5.1, the manufacturer shall perform risk analysis for the particular medical device as described in clauses 5.2 to 5.5. Here is how a Preliminary Hazard Analysis (PHA) can help.
Read MoreExeed is happy to introduce our new webinar series, Sharing Knowledge, Solving Problems, hosted by Dr. Naveen Agarwal. These interactive webinars are an opportunity for the medical device community to come together and discuss questions and obstacles surrounding ISO 14971 and risk management.
Read MoreClause 4.2 of ISO 14971:2019 requires the top management to define and document a policy for establishing criteria for risk acceptability. This policy must provide a framework to ensure that criteria are based on applicable national or regional regulations and relevant International Standards, stakeholder concerns and generally acknowledged state of the art.
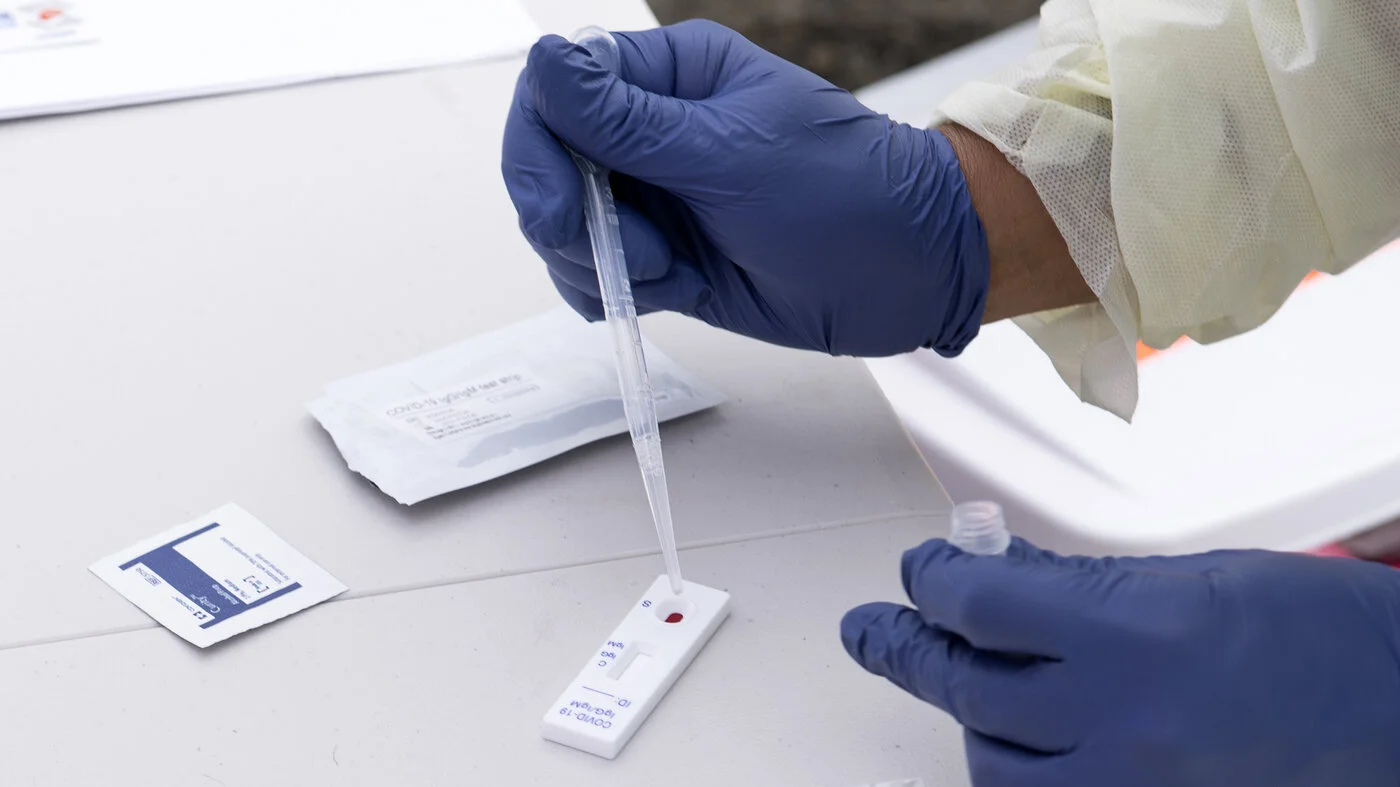
Read MoreOne of the challenges in controlling the rate of new infections during the COVID-19 pandemic has been the lack of a diagnostic test specifically indicated for asymptomatic patients. According to some estimates, nearly 40% of the new cases of SARS-CoV-2 infections do not have any visible symptoms. As a result, many of those infected are not aware they are carrying the virus and may be inadvertently adding to the rising rates of new infections. A newly authorized diagnostic test for asymptomatic patients could help.
Read MoreThe current COVID-19 global pandemic continues to remain out of control. As we write this blog, over 60,000 new infections are being reported on a daily basis in the United States, with a cumulative total of over 4 million cases and over 150,000 deaths reported since the pandemic first began here.
Read MoreThere is a lot of conversation about testing. Antibody tests, also called serological tests, help detect the presence of antibodies in blood samples. Antibody testing, when done at large scale, can help us understand the true rates of infection, mortality rates and level of immunity in the population on a regional basis. That is why these tests are a very powerful tool to help fight this devastating disease.
Read MoreThe medical landscape is constantly changing, particularly during the COVID-19 pandemic. With so much happening, there is a lot of confusion, particularly surrounding shortages of Personal Protective Equipment (PPE) and Face Masks. The FDA is consistently updating its Guidance and policy for various Face Masks, Face Shields and Respirators as new information becomes available. In this article, we will cover Face Shields and Respirators.
Read MoreSince the onset of the COVID-19 pandemic, there has been a lot of conversation surrounding the shortages of Personal Protective Equipment (PPE). In particular, we hear a lot about face masks and how they can help prevent the spread of COVID-19. But there is a lot of confusion out there - and many strong opinions - both for and against the use of face masks! In this article, we explain the FDA requirements, and guidance for face masks when they are used for a medical purpose such as diagnosis, or cure, mitigation, treatment of prevention of disease.
Read MoreShortages of N95 masks are forcing desperate measures. Decontamination of masks for multiple uses is a viable, low-risk-option under these extreme circumstances.
Read MoreMarket for interconnected medical devices is projected to grow but the regulatory pathway is still evolving. A new FDA guidance offers helpful recommendations for design and premarket submission.
Read MoreTimely MDR reporting is a challenging regulatory requirement for the industry. A new FDA guidance provides much-needed relief during a pandemic.
Read MoreIndustry is rapidly developing novel coronavirus diagnostic tests for emergency use authorization by the FDA.
Read MoreCybersecurity of medical devices is a rapidly evolving area of concern. Here is an update on recent FDA activities.
Read MoreGlobal outbreak of the recent novel coronavirus needs an all-hands-on-deck approach. Here is how QA/RA professionals in the medical industry can help.
Read MoreInteroperability holds the key to interconnected medical devices. A recent De Novo classification of a software for diabetes management shows the way forward.
Read MoreThese terms are foundational to risk analysis, yet they are poorly understood and often incorrectly applied.
Read More